Protein-coding gene in the species Homo sapiens
| PADI4 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
1WD8, 1WD9, 1WDA, 2DEW, 2DEX, 2DEY, 2DW5, 3APM, 3APN, 3B1T, 3B1U, 4DKT, 4X8C, 4X8G |
|
|
| Identifiers |
|---|
| Aliases | PADI4, PAD, PAD4, PADI5, PDI4, PDI5, peptidyl arginine deiminase 4 |
|---|
| External IDs | OMIM: 605347; MGI: 1338898; HomoloGene: 7883; GeneCards: PADI4; OMA:PADI4 - orthologs |
|---|
| EC number | 3.5.3.15 |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 1 (human)[1] |
|---|
| | Band | 1p36.13 | Start | 17,308,195 bp[1] |
|---|
| End | 17,364,004 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 4 (mouse)[2] |
|---|
| | Band | 4 D3|4 72.34 cM | Start | 140,473,176 bp[2] |
|---|
| End | 140,501,547 bp[2] |
|---|
|
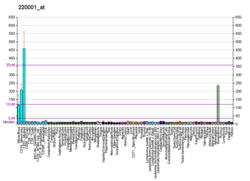
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - blood
- bone marrow
- bone marrow cells
- monocyte
- granulocyte
- spleen
- upper lobe of left lung
- testicle
- right lung
- gonad
|
| | Top expressed in | - granulocyte
- morula
- lip
- hair follicle
- tibiofemoral joint
- cervix
- bone marrow
- otic vesicle
- embryo
- blood
|
| | More reference expression data |
|
|---|
| BioGPS | 

 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - metal ion binding
- arginine deiminase activity
- protein binding
- hydrolase activity
- protein-arginine deiminase activity
- calcium ion binding
- arginine binding
- protein homodimerization activity
| | Cellular component | - nucleoplasm
- nucleus
- cytosol
- cytoplasm
- protein-containing complex
| | Biological process | - chromatin remodeling
- nucleosome assembly
- regulation of transcription, DNA-templated
- immune system process
- transcription, DNA-templated
- stem cell population maintenance
- innate immune response
- chromatin organization
- arginine deiminase pathway
| | Sources:Amigo / QuickGO |
|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Protein-arginine deiminase type-4, is a human protein which in humans is encoded by the PADI4 gene.[5][6] The protein as an enzyme, specifically protein-arginine deiminase, a type of hydrolase.
Molecular biology
The human gene is found on the short arm of Chromosome 1 near the telomere (1p36.13). It is located on the Watson (plus) strand and is 55,806 bases long. The protein is 663 amino acids long with a molecular weight of 74,095 Da.[5]
Function
This gene is a member of a gene family which encodes enzymes responsible for the conversion of arginine to citrulline residues (citrullination). This gene may play a role in granulocyte and macrophage development leading to inflammation and immune response.[6] PADI4 plays a role in the epigenetics,[7] the deimination of arginines on histones H3 and H4 can act antagonistically to arginine methylation.[8]
The protein may be found in oligomers and binds 5 calcium ions per subunit. It catalyses the reaction:
- Protein L-arginine + H2O = protein L-citrulline + NH3
Subcellular and tissue distribution
It is normally found in the cytoplasm, nucleus and in cytoplasmic granules of eosinophils and neutrophils. It is not expressed in peripheral monocytes or lymphocytes. It is also expressed in rheumatoid arthritis synovial tissues.
References
- ^ a b c ENSG00000159339 GRCh38: Ensembl release 89: ENSG00000280908, ENSG00000159339 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000025330 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Nakashima K, Hagiwara T, Ishigami A, Nagata S, Asaga H, Kuramoto M, et al. (September 1999). "Molecular characterization of peptidylarginine deiminase in HL-60 cells induced by retinoic acid and 1alpha,25-dihydroxyvitamin D(3)". The Journal of Biological Chemistry. 274 (39): 27786–27792. doi:10.1074/jbc.274.39.27786. PMID 10488123.
- ^ a b "Entrez Gene: PADI4 peptidyl arginine deiminase, type IV".
- ^ Sams KL, Mukai C, Marks BA, Mittal C, Demeter EA, Nelissen S, et al. (October 2022). "Delayed puberty, gonadotropin abnormalities and subfertility in male Padi2/Padi4 double knockout mice". Reproductive Biology and Endocrinology. 20 (1): 150. doi:10.1186/s12958-022-01018-w. PMC 9555066. PMID 36224627.
- ^ Kouzarides T (February 2007). "Chromatin modifications and their function". Cell. 128 (4): 693–705. doi:10.1016/j.cell.2007.02.005. PMID 17320507.
Further reading
- Nakashima K, Hagiwara T, Ishigami A, Nagata S, Asaga H, Kuramoto M, et al. (September 1999). "Molecular characterization of peptidylarginine deiminase in HL-60 cells induced by retinoic acid and 1alpha,25-dihydroxyvitamin D(3)". The Journal of Biological Chemistry. 274 (39): 27786–27792. doi:10.1074/jbc.274.39.27786. PMID 10488123.
- Zhang QH, Ye M, Wu XY, Ren SX, Zhao M, Zhao CJ, et al. (October 2000). "Cloning and functional analysis of cDNAs with open reading frames for 300 previously undefined genes expressed in CD34+ hematopoietic stem/progenitor cells". Genome Research. 10 (10): 1546–1560. doi:10.1101/gr.140200. PMC 310934. PMID 11042152.
- Asaga H, Nakashima K, Senshu T, Ishigami A, Yamada M (July 2001). "Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils". Journal of Leukocyte Biology. 70 (1): 46–51. doi:10.1189/jlb.70.1.46. PMID 11435484. S2CID 11448270.
- Nakashima K, Hagiwara T, Yamada M (December 2002). "Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes". The Journal of Biological Chemistry. 277 (51): 49562–49568. doi:10.1074/jbc.M208795200. PMID 12393868.
- Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, et al. (August 2003). "Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis". Nature Genetics. 34 (4): 395–402. doi:10.1038/ng1206. PMID 12833157. S2CID 20136298.
- Barton A, Bowes J, Eyre S, Spreckley K, Hinks A, John S, Worthington J (April 2004). "A functional haplotype of the PADI4 gene associated with rheumatoid arthritis in a Japanese population is not associated in a United Kingdom population". Arthritis and Rheumatism. 50 (4): 1117–1121. doi:10.1002/art.20169. PMID 15077293.
- Chavanas S, Méchin MC, Takahara H, Kawada A, Nachat R, Serre G, Simon M (April 2004). "Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6". Gene. 330: 19–27. doi:10.1016/j.gene.2003.12.038. PMID 15087120.
- Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M (August 2004). "Structural basis for Ca(2+)-induced activation of human PAD4". Nature Structural & Molecular Biology. 11 (8): 777–783. doi:10.1038/nsmb799. PMID 15247907. S2CID 11947135.
- Hoppe B, Heymann GA, Tolou F, Kiesewetter H, Doerner T, Salama A (November 2004). "High variability of peptidylarginine deiminase 4 (PADI4) in a healthy white population: characterization of six new variants of PADI4 exons 2-4 by a novel haplotype-specific sequencing-based approach". Journal of Molecular Medicine. 82 (11): 762–767. doi:10.1007/s00109-004-0584-6. PMID 15338034. S2CID 8356741.
- Cuthbert GL, Daujat S, Snowden AW, Erdjument-Bromage H, Hagiwara T, Yamada M, et al. (September 2004). "Histone deimination antagonizes arginine methylation". Cell. 118 (5): 545–553. doi:10.1016/j.cell.2004.08.020. PMID 15339660. S2CID 8948511.
- Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, et al. (October 2004). "Human PAD4 regulates histone arginine methylation levels via demethylimination". Science. 306 (5694): 279–283. Bibcode:2004Sci...306..279W. doi:10.1126/science.1101400. PMID 15345777. S2CID 1579362.
- Nakayama-Hamada M, Suzuki A, Kubota K, Takazawa T, Ohsaka M, Kawaida R, et al. (February 2005). "Comparison of enzymatic properties between hPADI2 and hPADI4". Biochemical and Biophysical Research Communications. 327 (1): 192–200. doi:10.1016/j.bbrc.2004.11.152. PMID 15629448.
- Lee YH, Coonrod SA, Kraus WL, Jelinek MA, Stallcup MR (March 2005). "Regulation of coactivator complex assembly and function by protein arginine methylation and demethylimination". Proceedings of the National Academy of Sciences of the United States of America. 102 (10): 3611–3616. Bibcode:2005PNAS..102.3611L. doi:10.1073/pnas.0407159102. PMC 553305. PMID 15731352.
- Kearney PL, Bhatia M, Jones NG, Yuan L, Glascock MC, Catchings KL, et al. (August 2005). "Kinetic characterization of protein arginine deiminase 4: a transcriptional corepressor implicated in the onset and progression of rheumatoid arthritis". Biochemistry. 44 (31): 10570–10582. doi:10.1021/bi050292m. PMID 16060666.
- Ikari K, Kuwahara M, Nakamura T, Momohara S, Hara M, Yamanaka H, et al. (October 2005). "Association between PADI4 and rheumatoid arthritis: a replication study". Arthritis and Rheumatism. 52 (10): 3054–3057. doi:10.1002/art.21309. PMID 16200584.
- Chang X, Han J (March 2006). "Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors". Molecular Carcinogenesis. 45 (3): 183–196. doi:10.1002/mc.20169. PMID 16355400. S2CID 40916607.
- Plenge RM, Padyukov L, Remmers EF, Purcell S, Lee AT, Karlson EW, et al. (December 2005). "Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4". American Journal of Human Genetics. 77 (6): 1044–1060. doi:10.1086/498651. PMC 1285162. PMID 16380915.
- Hoppe B, Häupl T, Gruber R, Kiesewetter H, Burmester GR, Salama A, Dörner T (2006). "Detailed analysis of the variability of peptidylarginine deiminase type 4 in German patients with rheumatoid arthritis: a case-control study". Arthritis Research & Therapy. 8 (2): R34. doi:10.1186/ar1889. PMC 1526594. PMID 16469113.
PDB gallery
-
1wd8: Calcium free form of human peptidylarginine deiminase type4 (PAD4) -
1wd9: Calcium bound form of human peptidylarginine deiminase type4 (PAD4) -
1wda: Crystal structure of human peptidylarginine deiminase type4 (PAD4) in complex with benzoyl-L-arginine amide -
2dew: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H3 N-terminal tail including Arg8 -
2dex: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H3 N-terminal peptide including Arg17 -
2dey: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H4 N-terminal tail including Arg3 -
2dw5: Crystal structure of human peptidylarginine deiminase 4 in complex with N-alpha-benzoyl-N5-(2-fluoro-1-iminoethyl)-L-ornithine amide |

 1wd8: Calcium free form of human peptidylarginine deiminase type4 (PAD4)
1wd8: Calcium free form of human peptidylarginine deiminase type4 (PAD4) 1wd9: Calcium bound form of human peptidylarginine deiminase type4 (PAD4)
1wd9: Calcium bound form of human peptidylarginine deiminase type4 (PAD4) 1wda: Crystal structure of human peptidylarginine deiminase type4 (PAD4) in complex with benzoyl-L-arginine amide
1wda: Crystal structure of human peptidylarginine deiminase type4 (PAD4) in complex with benzoyl-L-arginine amide 2dew: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H3 N-terminal tail including Arg8
2dew: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H3 N-terminal tail including Arg8 2dex: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H3 N-terminal peptide including Arg17
2dex: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H3 N-terminal peptide including Arg17 2dey: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H4 N-terminal tail including Arg3
2dey: Crystal structure of human peptidylarginine deiminase 4 in complex with histone H4 N-terminal tail including Arg3 2dw5: Crystal structure of human peptidylarginine deiminase 4 in complex with N-alpha-benzoyl-N5-(2-fluoro-1-iminoethyl)-L-ornithine amide
2dw5: Crystal structure of human peptidylarginine deiminase 4 in complex with N-alpha-benzoyl-N5-(2-fluoro-1-iminoethyl)-L-ornithine amide
























