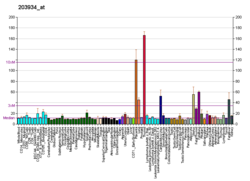
Kinase insert domain receptor
| KDR | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | KDR, CD309, FLK1, VEGFR, VEGFR2, Kinase insert domain receptor | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 191306; MGI: 96683; HomoloGene: 55639; GeneCards: KDR; OMA:KDR - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Kinase insert domain receptor (KDR, a type IV receptor tyrosine kinase) also known as vascular endothelial growth factor receptor 2 (VEGFR-2) is a VEGF receptor. KDR is the human gene encoding it. KDR has also been designated as CD309 (cluster of differentiation 309). KDR is also known as Flk1 (Fetal Liver Kinase 1).
The Q472H germline KDR genetic variant affects VEGFR-2 phosphorylation and has been found to associate with microvessel density in NSCLC.[5]
Interactions
Kinase insert domain receptor has been shown to interact with SHC2,[6] Annexin A5[7] and SHC1.[8][9]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000128052 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000062960 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Glubb DM, Cerri E, Giese A, Zhang W, Mirza O, Thompson EE, Chen P, Das S, Jassem J, Rzyman W, Lingen MW, Salgia R, Hirsch FR, Dziadziuszko R, Ballmer-Hofer K, Innocenti F (August 2011). "Novel functional germline variants in the VEGF receptor 2 gene and their effect on gene expression and microvessel density in lung cancer". Clinical Cancer Research. 17 (16): 5257–67. doi:10.1158/1078-0432.CCR-11-0379. PMC 3156871. PMID 21712447.
- ^ Warner AJ, Lopez-Dee J, Knight EL, Feramisco JR, Prigent SA (April 2000). "The Shc-related adaptor protein, Sck, forms a complex with the vascular-endothelial-growth-factor receptor KDR in transfected cells". The Biochemical Journal. 347 (Pt 2): 501–9. doi:10.1042/0264-6021:3470501. PMC 1220983. PMID 10749680.
- ^ Wen Y, Edelman JL, Kang T, Sachs G (May 1999). "Lipocortin V may function as a signaling protein for vascular endothelial growth factor receptor-2/Flk-1". Biochemical and Biophysical Research Communications. 258 (3): 713–21. doi:10.1006/bbrc.1999.0678. PMID 10329451.
- ^ Zanetti A, Lampugnani MG, Balconi G, Breviario F, Corada M, Lanfrancone L, Dejana E (April 2002). "Vascular endothelial growth factor induces SHC association with vascular endothelial cadherin: a potential feedback mechanism to control vascular endothelial growth factor receptor-2 signaling". Arteriosclerosis, Thrombosis, and Vascular Biology. 22 (4): 617–22. doi:10.1161/01.ATV.0000012268.84961.AD. PMID 11950700.
- ^ D'Angelo G, Martini JF, Iiri T, Fantl WJ, Martial J, Weiner RI (May 1999). "16K human prolactin inhibits vascular endothelial growth factor-induced activation of Ras in capillary endothelial cells". Molecular Endocrinology. 13 (5): 692–704. doi:10.1210/mend.13.5.0280. PMID 10319320.
Further reading
- Holmes K, Roberts OL, Thomas AM, Cross MJ (October 2007). "Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition". Cellular Signalling. 19 (10): 2003–12. doi:10.1016/j.cellsig.2007.05.013. PMID 17658244.
- Petrova TV, Makinen T, Alitalo K (November 1999). "Signaling via vascular endothelial growth factor receptors". Experimental Cell Research. 253 (1): 117–30. doi:10.1006/excr.1999.4707. PMID 10579917.
- Wang J, Fu X, Jiang C, Yu L, Wang M, Han W, Liu L, Wang J (May 2014). "Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signaling pathway in a rat model of vascular dementia". Behavioural Brain Research. 265: 171–80. doi:10.1016/j.bbr.2014.02.033. PMC 4000455. PMID 24589546.
- Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K, Shibuya M (May 2000). "Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction". Annals of the New York Academy of Sciences. 902 (1): 201–5, discussion 205–7. Bibcode:2000NYASA.902..201S. doi:10.1111/j.1749-6632.2000.tb06314.x. PMID 10865839. S2CID 11488629.
- Zachary I, Gliki G (February 2001). "Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family". Cardiovascular Research. 49 (3): 568–81. doi:10.1016/S0008-6363(00)00268-6. PMID 11166270.
- Vené R, Benelli R, Noonan DM, Albini A (2001). "HIV-Tat dependent chemotaxis and invasion, key aspects of tat mediated pathogenesis". Clinical & Experimental Metastasis. 18 (7): 533–8. doi:10.1023/A:1011991906685. PMID 11688957. S2CID 24787929.
- Lenton K (2003). "VEGFR-2 (KDR/Flk-1)". Journal of Biological Regulators and Homeostatic Agents. 16 (3): 227–32. PMID 12456025.
- Matsumoto T, Mugishima H (June 2006). "Signal transduction via vascular endothelial growth factor (VEGF) receptors and their roles in atherogenesis". Journal of Atherosclerosis and Thrombosis. 13 (3): 130–5. doi:10.5551/jat.13.130. PMID 16835467.
External links
- Kinase+insert+domain+receptor at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P35968 (Vascular endothelial growth factor receptor 2) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
- v
- t
- e
-
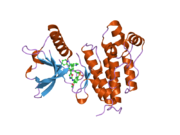 1y6a: Crystal structure of VEGFR2 in complex with a 2-anilino-5-aryl-oxazole inhibitor
1y6a: Crystal structure of VEGFR2 in complex with a 2-anilino-5-aryl-oxazole inhibitor -
 1y6b: Crystal structure of VEGFR2 in complex with a 2-anilino-5-aryl-oxazole inhibitor
1y6b: Crystal structure of VEGFR2 in complex with a 2-anilino-5-aryl-oxazole inhibitor
 | This transmembrane receptor-related article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e